Mapping
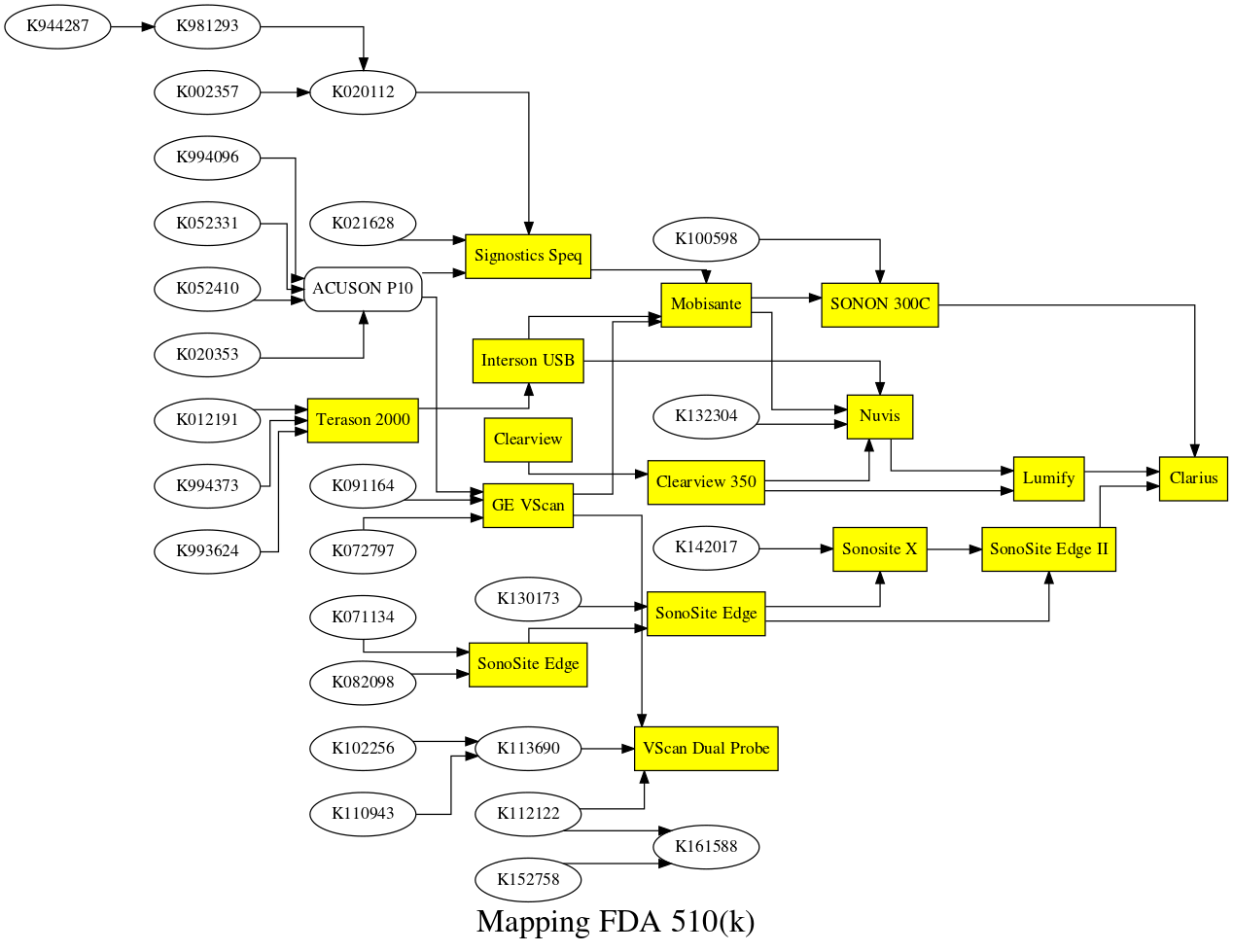
All FDA requests for approval are available on the FDA website. To ask for an approval, the entity requesting the approval base themselves on previous devices, the "predicates". It's therefore quite easy to map the predicates graph ... see above.
List of probes
Acuson
- Name: ACUSON P10 Ultrasound System
- URL: https://www.accessdata.fda.gov/cdrh_docs/pdf6/K063761.pdf
Predicates:
- K052331
- K063761
- K052410
- K020353
- K994096
Signostics Speq
- Name: Signostics Speq
- URL: https://www.accessdata.fda.gov/cdrh_docs/pdf9/K090505.pdf
Predicates:
- K020112
- K063761
- K021628
GE Vscan Extend
- Name: GE Vscan Extend
- URL: https://www.accessdata.fda.gov/cdrh_docs/pdf16/K161588.pdf
Predicates:
- K152758
- K112122
VScan Dual Probe
- Name: VScan Dual Probe
- URL: https://www.accessdata.fda.gov/cdrh_docs/pdf14/K140693.pdf
Predicates:
- K092756
- K113690
- K112122
Mobisante
- Name: MobiUS
- URL: https://www.accessdata.fda.gov/cdrh_docs/pdf10/K102153.pdf
Predicates:
- K070907
- K092756
- K090505
Interson USB
- Name: Interson USB
- URL: https://www.accessdata.fda.gov/cdrh_docs/pdf7/K070907.pdf
Predicates:
- K030191
Terason Model 2000
- Name: Terason Model 2000
- URL: https://www.accessdata.fda.gov/cdrh_docs/pdf3/K030191.pdf
Predicates:
- K012191
- K994373
- K993624
Clarius Ultrasound System
- Name: Clarius Ultrasound System
- URL: http://www.accessdata.fda.gov/cdrh_docs/pdf16/K163138.pdf
Predicates:
- K151339
- K152899
- K153626
Lumify
- Name: Lumify
- URL: https://www.accessdata.fda.gov/cdrh_docs/pdf15/K152899.pdf
Predicates:
- K133833
- K120321
Nuvis
- Name: Nuvis
- URL: https://www.accessdata.fda.gov/cdrh_docs/pdf13/K133833.pdf
Predicates:
- K120321
- K132304
- K070907
- K102153
Clearview
- Name: Clearview
- URL: https://www.accessdata.fda.gov/cdrh_docs/pdf12/K120321.pdf
Predicates:
- K043535